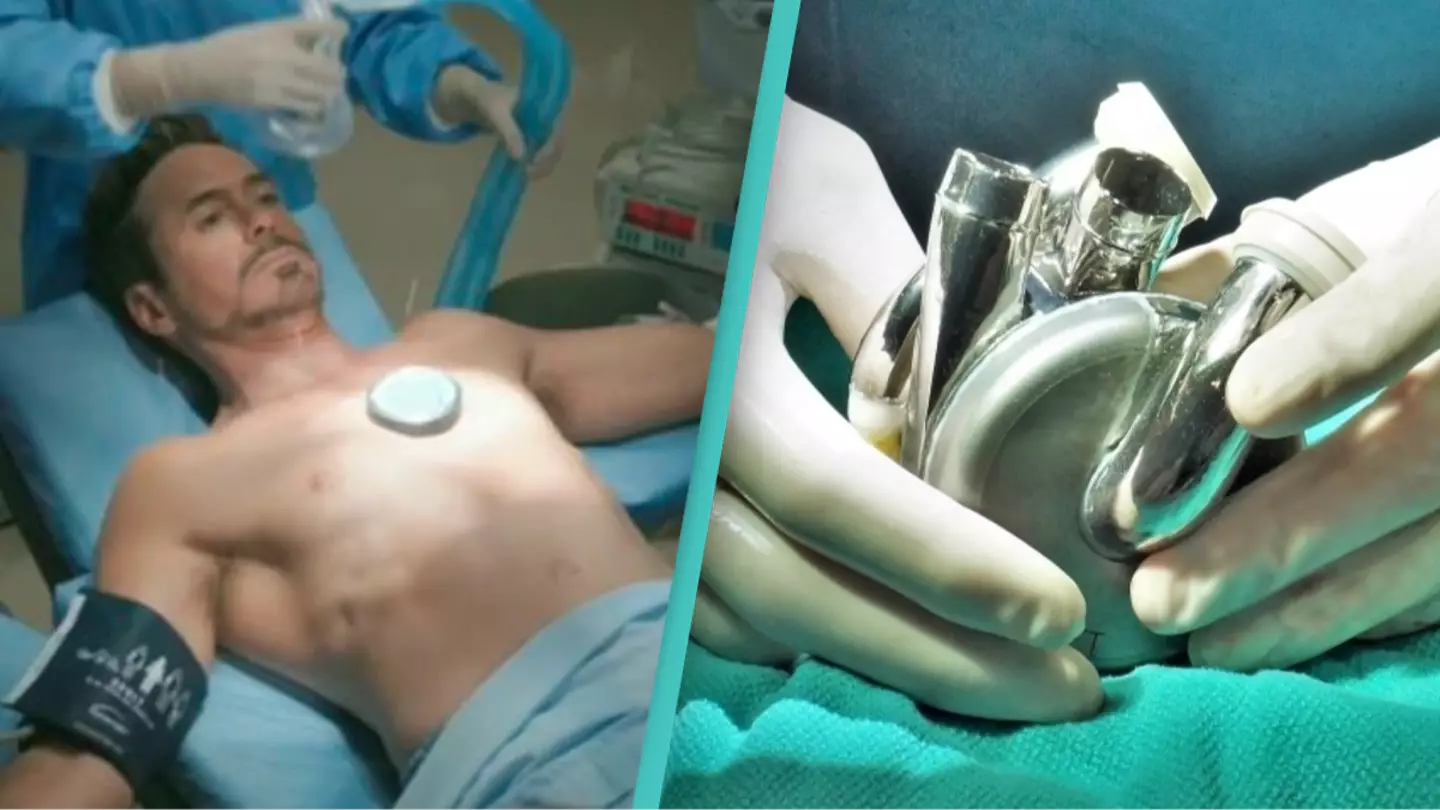
A clinical trial's team successfully implanted a 'valveless artificial heart' in a human patient with 'end-stage heart failure'.
With patients in need of a heart transplant reported as potentially waiting around three years, clinical-stage medical device company BiVACOR - in partnership with the Texas Heart Institute and Baylor St. Lukes Medical Center - created a totally artificial heart, which uses the same magnetic levitation (maglev) technology as high-speed rail lines.
And on July 9, 2024, a 57-year-old man became the first ever patient with end-stage heart failure to have the totally artificial maglev heart implanted.
The device
The heart was designed to act as a stand-in 'for patients living with severe biventricular heart failure or univentricular heart failure in which left ventricular assist device support is not recommended,' pumping blood around the body until the patient receives a new heart.
Advert
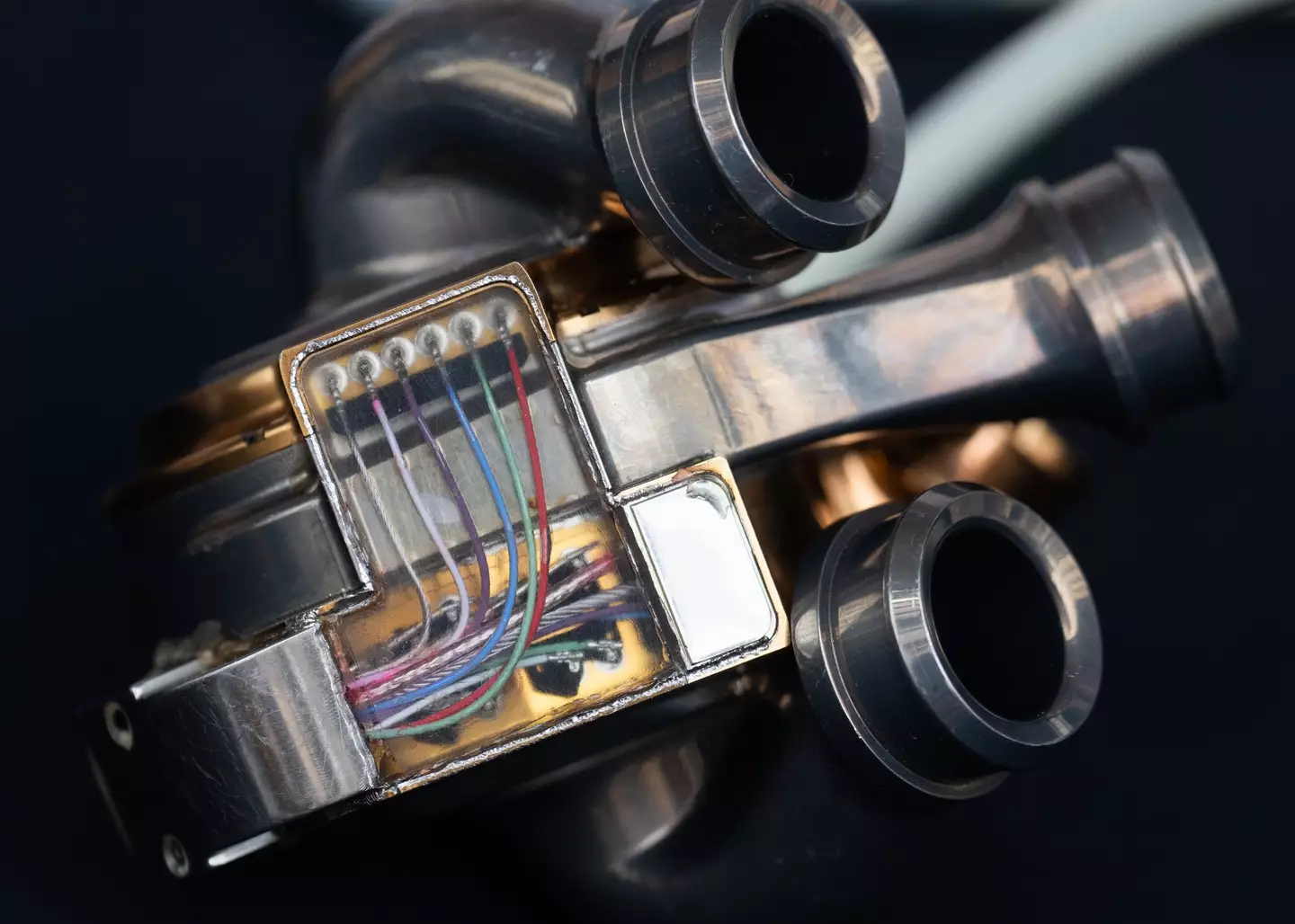
The titanium artificial heart - made by BiVACOR, called the BiVACOR Total Artificial Heart (TAH) - has a body surface area of 1.4m2, BiVACOR's website explains.
It's suitable for 'most men and women' and 'is capable of providing enough cardiac output for an adult male undergoing exercise'.
The artificial heart is valveless, instead featuring a pump design 'with a single moving part', which uses maglev technology - a magnetically suspended dual-sided rota 'with left and right vanes positioned within two separate pump chambers, forming a double-sided centrifugal impeller that propels blood from the respective pump chambers to the pulmonary (lung) and systemic (body) circulations'.
The non-contact suspension is 'designed to eliminate the potential for mechanical wear and provide large blood gaps that minimize blood trauma, offering a durable, reliable, and biocompatible heart replacement'.
Basically, the device 'replaces both ventricles of a failing heart' and keeps blood pumping round the body improving a patient's chance of survival, while they wait for a new heart.
The procedure
The trial was approved by the US Food and Drug Administration (FDA) as an early feasibility study (EFS) - 'a limited clinical investigation of a device early in development,' the FDA's website explains.
It aimed to 'evaluate the safety and performance of the BiVACOR TAH as a bridge-to-transplant solution for patients with severe biventricular heart failure or univentricular heart failure in which left ventricular assist device support is not recommended,' Clinical Trials Arena notes.
And so earlier this month, the device was implanted into the 57-year-old patient - and it was a success. While it's not the first artificial heart to be successfully implanted, it's the first to use maglev technology.
A donor heart later became available for the patient on 17 July.
The results and future plans
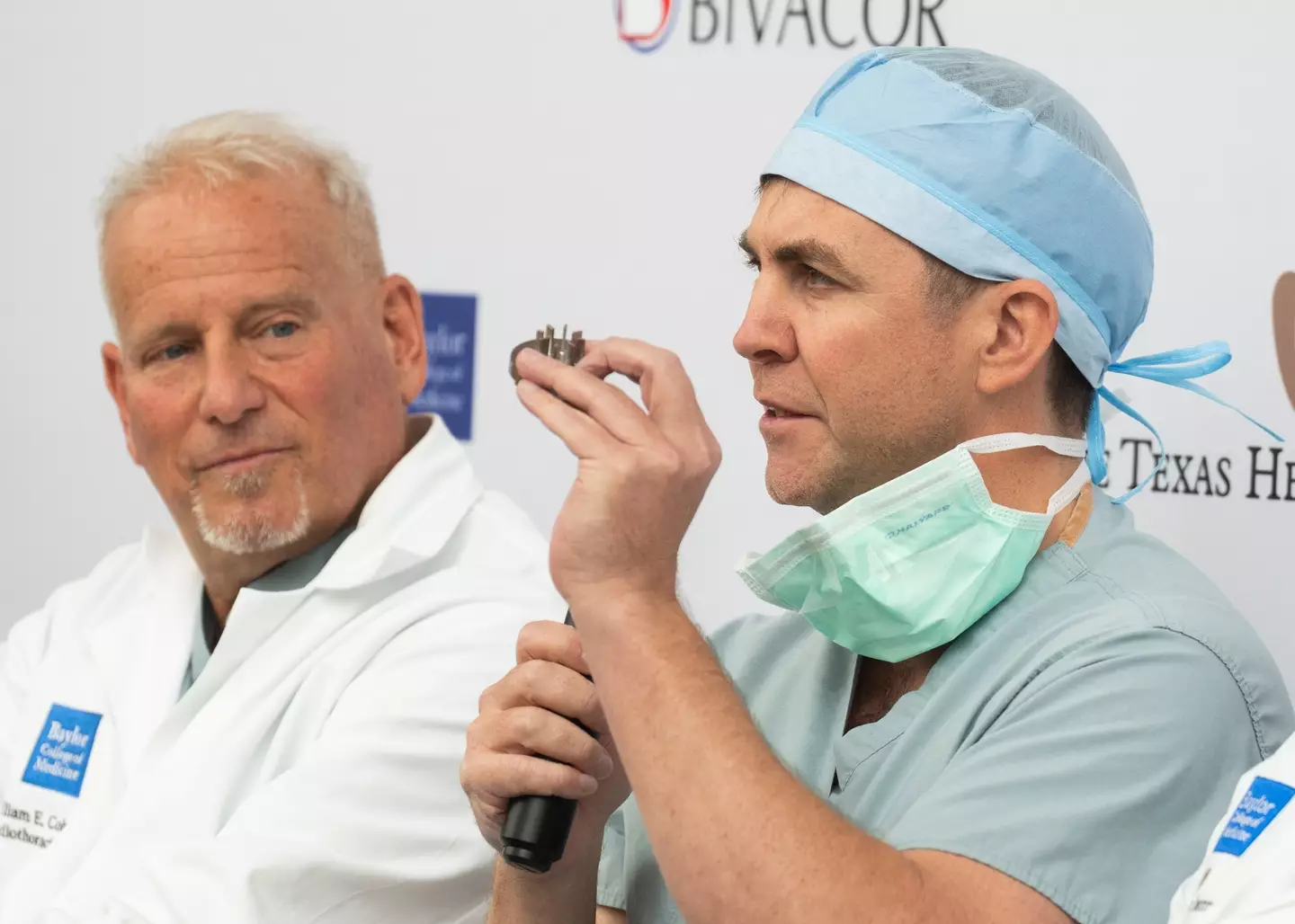
Given the successful implantation of the first ever human patient, four other patients are now set to be enrolled in the study.
Surgical director of heart transplantation at Baylor St Luke’s Medical Center, Alexis Shafii, said: "This device may serve as a life-saving bridge to a heart transplant; future studies may prove its potential as a long-term pump that can effectively serve as a total replacement for a patient’s heart.
"We anticipate the BiVACOR TAH may eventually save numerous lives, as well as improve the quality of life for patients who otherwise have no alternative therapy available."
Founder and CTO of BiVACOR, Daniel Timms said, as per New Atlas: "This achievement would not have been possible without the courage of our first patient and their family, the dedication of our team, and our expert collaborators at The Texas Heart Institute.
"Utilizing advanced maglev technology, our TAH brings us one step closer to providing a desperately needed option for people with end-stage heart failure who require support while waiting for a heart transplant. I look forward to continuing the next phase of our clinical trial."
Topics: Science, Texas, US News, World News, Health, Technology